
30th March, 2023 - Press notes
Adding a peptide, a molecule made of several amino acids, to oxaliplatin, a chemotherapy drug used to treat colorectal cancer, avoids the side effects this treatment can have on normal cells, including potential chemotherapy resistance.
This is confirmed by an article published in the Journal of Medicinal Chemistry, led by the Hospital del Mar Medical Research Institute and the Institute for Research in Biomedicine (IRB Barcelona). This work is the second part of a recent study that explored how platinum accumulation in normal cells promotes platinum resistance in colorectal cancer cells.
This new approach prevents healthy cells surrounding the tumour from accumulating platinum. This avoids the activation of certain genes linked to poor treatment response and tumour progression.
A multidisciplinary team of scientists from the Hospital del Mar Medical Research Institute (IMIM-Hospital del Mar) and the Institute for Research in Biomedicine (IRB Barcelona) has led a study, recently published in the Journal of Medicinal Chemistry, which proposes a therapeutic approach for preventing the development of resistance to chemotherapy with oxaliplatin, one of the standard treatments for colorectal cancer. The work, which also involved clinicians from the Pathological Anatomy and Medical Oncology departments at Hospital del Mar, as well as researchers from the University of Oviedo and the CIBER on Cancer (CIBERONC), is a further step towards personalising the therapeutic approach to cancer.

Group members
In a study recently published in Nature Communications, these researchers had already shown how this type of chemotherapy accumulates in the healthy cells surrounding the cancer cells, the fibroblasts. This causes the activation of a number of genes linked to poor treatment response and tumour progression, which stimulates the tumour environment and helps cancer cells that survived chemotherapy to proliferate once again. To prevent this, the authors of this new study propose conjugating a cell-penetrating peptide to oxaliplatin.
"By incorporating this peptide, we can change a systemic therapy which impacts both healthy organs and the tumour microenvironment, into a targeted therapy that is more focused, bringing us one step closer to personalized medicine.", points out Dr. Alexandre Calon, researcher at the IMIM-Hospital del Mar and co-leader of the study. The conclusions are based on the analysis of tumour samples from nearly 200 colorectal cancer patients. In addition, mice tumours and patients tissues treated ex vivo were analysed to show that adding a specific peptide to oxaliplatin reduced the adverse effects of this chemotherapy on non-cancerous cells in the tumour and could therefore reduce resistance to this treatment.
The results indicate that platinum accumulation in the tumour microenvironment in mice treated with this new approach drops dramatically and is up to 3.5 times lower. "We have seen that the chemotherapy load is reduced in fibroblasts treated with the new compound compared to those treated with oxaliplatin. This reduces the possibility of inducing tumour cell treatment resistance", explains Dr. Calon. It is therefore important to bear in mind that "a tumour is not only composed of cancer cells, but contains also a microenvironment made of blood vessels, fibroblasts and immune cells, which are there to structure the tumour".
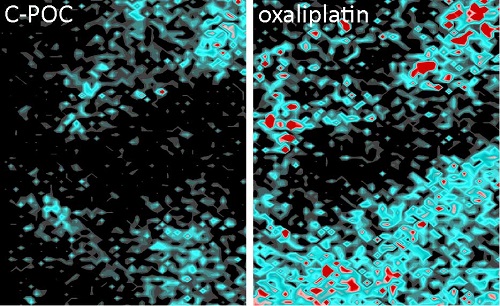
Platinum accumulation after treatment
The researchers also determined that the new drug not only accumulates less in the tumour microenvironment, but also in the organs that are normally most heavily affected by chemotherapy, such as the colon itself, the kidneys and the liver. In this sense, as Dr. Jenniffer Linares, first author of the study, explains "Standard treatment has a series of side effects on the patient, which we think could be reduced with the new drug, as less platinum accumulates in healthy tissues."
"This study is a crucial first step for the future clinical development of treatments that cause fewer side effects and are more effective in patients with colorectal cancer, taking into consideration the fact that the normal cells involved in tumours play a key role in treatment efficacy", explains Dr. Clara Montagut, head of the gastrointestinal tumour unit at the Hospital del Mar and a CIBERONC researcher.
These findings prompted Dr. Daniele Lo Re, who co-led the study, to observe that "By chemically modifying the structure of oxaliplatin, we can modulate its activity in the tumour microenvironment without any loss of efficacy against cancer cells. From this point on, we can think about integrating this approach into drug discovery processes, using appropriate cellular models that allow us to validate a greater number of potential new drugs both in the tumour and in its microenvironment."
The work was supported by the Carlos III Health Institute and the Spanish Association Against Cancer.
Peptide-Platinum(IV) Conjugation Minimizes the Negative Impact of Current Anticancer Chemotherapy on Nonmalignant Cells. Jenniffer Linares, Monica Varese, Anna Sallent-Aragay, Ana Méndez, Sergio Palomo-Ponce, Mar Iglesias, Eduard Batlle, Jorge Pisonero, Clara Montagut, Ernest Giralt, Daniele Lo Re, and Alexandre Calon. Journal of Medicinal Chemistry Article ASAP DOI: 10.1021/acs.jmedchem.2c01717
Linares, J., Sallent-Aragay, A., Badia-Ramentol, J. et al. Long-term platinum-based drug accumulation in cancer-associated fibroblasts promotes colorectal cancer progression and resistance to therapy. Nat Commun 14, 746 (2023). https://doi.org/10.1038/s41467-023-36334-1
Parc Salut Mar
Passeig Marítim 25-29 Barcelona 08003
See location on Google maps
Phone: 93 248 30 00 · Fax: 93 248 32 54
Information request
© 2006 - 2025 Hospital del Mar · Legal notice and Privacy Police | Cookie Policy | Accessibility